Comparison of biofilm models for producing artificial active white spot lesions
DOI:
https://doi.org/10.1590/1678-7757-2023-045Keywords:
Dental caries, Dental enamel, Biofilms, Microbiology, Raman spectrum analysisAbstract
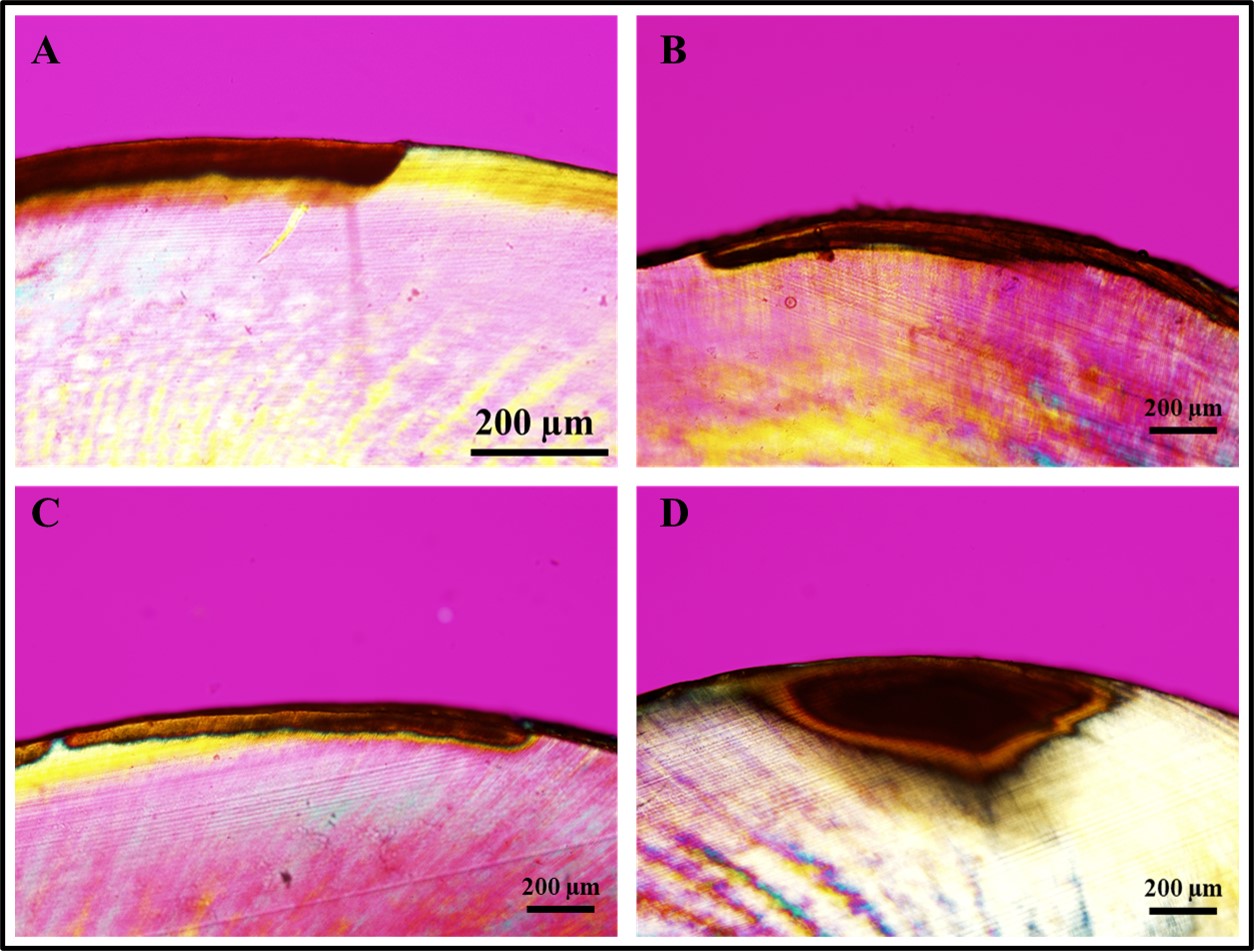
Creating artificial caries-like lesions that mimic the complex changes observed in natural caries is essential for properly evaluating new strategies, dental materials, and devices designed to arrest their progression and avoid more costly and invasive treatments. Objective: This study compared three protocols for developing artificial white spot lesions (WSL) using biofilm models. Methodology: In total, 45 human enamel specimens were sterilized and allocated into three groups based on the biofilm model: Streptococcus sobrinus and Lactobacillus casei (Ss+Lc), Streptococcus sobrinus (Ss), or Streptococcus mutans (Sm). Specimens were incubated in filter-sterilized human saliva to form the acquired pellicle and then subjected to the biofilm challenge consisting of three days of incubation with bacteria (for demineralization) and one day of remineralization, which was performed once for Ss+Lc (four days total), four times for Ss (16 days total), and three times for Sm (12 days total). After WSL creation, the lesion fluorescence, depth, and chemical composition were assessed using Quantitative Light-induced Fluorescence (QLF), Polarized Light Microscopy (PLM), and Raman Spectroscopy, respectively. Statistical analysis consisted of two-way ANOVA followed by Tukey’s post hoc test (α=0.05). WSL created using the Ss+Lc protocol presented statistically significant higher fluorescence loss (ΔF) and integrated fluorescence (ΔQ) in comparison to the other two protocols (p<0.001). Results: In addition, Ss+Lc resulted in significantly deeper WSL (137.5 µm), followed by Ss (84.1 µm) and Sm (54.9 µm) (p<0.001). While high mineral content was observed in sound enamel surrounding the WSL, lesions created with the Ss+Lc protocol showed the highest demineralization level and changes in the mineral content among the three protocols. Conclusion: The biofilm model using S. sobrinus and L. casei for four days was the most appropriate and simplified protocol for developing artificial active WSL with lower fluorescence, higher demineralization, and greater depth.
Downloads
References
Featherstone JD, Chaffee BW. The evidence for Caries Management by Risk Assessment (CAMBRA®). Adv Dent Res. 2018;29(1):9-14. doi: 10.1177/0022034517736500
Featherstone JD. Prevention and reversal of dental caries: role of low level fluoride. Community Dent Oral Epidemiol. 1999;27(1):31-40. doi: 10.1111/j.1600-0528.1999.tb01989.x
Kidd EA, Fejerskov O. What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. J Dent Res. 2004;83 Spec No C:C35-8. doi: 10.1177/154405910408301s07
Guzmán-Armstrong S, Chalmers J, Warren JJ. White spot lesions: prevention and treatment. Am J Orthod Dentofacial Orthop. 2010;138(6):690-6. doi: 10.1016/j.ajodo.2010.07.007
Yu OY, Zhao IS, Mei ML, Lo EC, Chu CH. A Review of the common models used in mechanistic studies on demineralization-remineralization for cariology research. Dent J (Basel). 2017;5(2):20. doi: 10.3390/dj5020020
Alkattan R, Lippert F, Tang Q, Eckert GJ, Ando M. The influence of hardness and chemical composition on enamel demineralization and subsequent remineralization. J Dent. 2018;75:34-40. doi: 10.1016/j.jdent.2018.05.002.
Shu M, Wong L, Miller JH, Sissons CH. Development of multi-species consortia biofilms of oral bacteria as an enamel and root caries model system. Arch Oral Biol. 2000;45(1):27-40. doi: 10.1016/s0003-9969(99)00111-9
Arthur RA, Kohara EK, Waeiss RA, Eckert GJ, Zero D, Ando M. Enamel carious lesion development in response to sucrose and fluoride concentrations and to time of biofilm formation: an artificial-mouth study. J Oral Dis. 2014;2014:348032. doi: 10.1155/2014/348032
Levy FM, Braga AS, Pelá VT, Lavender S, Zhang D, Pilch S, et al. Characterization of white spot lesions formed on human enamel under microcosm biofilm for different experimental periods. J Appl Oral Sci. 2022;30:e20210560. doi: 10.1590/1678-7757-2021-0560
Arthur RA, Waeiss RA, Hara AT, Lippert F, Eckert GJ, Zero DT. A defined-multispecies microbial model for studying enamel caries development. Caries Res. 2013;47(4):318-24. doi: 10.1159/000347050
Salli KM, Ouwehand AC. The use of in vitro model systems to study dental biofilms associated with caries: a short review. J Oral Microbiol. 2015;7:26149. doi: 10.3402/jom.v7.26149
Maske TT, Brauner KV, Nakanishi L, Arthur RA, van de Sande FH, Cenci MS. An in vitro dynamic microcosm biofilm model for caries lesion development and antimicrobial dose-response studies. Biofouling. 2016;32(3):339-48. doi: 10.1080/08927014.2015.1130824
Steiner-Oliveira C, Rodrigues LK, Zanin IC, Carvalho CL, Kamiya RU, Hara AT, et al. An in vitro microbial model associated with sucrose to produce dentin caries lesions. Cent Eur J Biol. 2011;6(3):414-21. doi: 10.2478/s11535-011-0011-2
Amaechi BT, Tenuta LMA, Ricomini Filho AP, Cury JA. Protocols to study dental caries in vitro: microbial caries models. Methods Mol Biol. 2019;1922:357-68. doi: 10.1007/978-1-4939-9012-2_32
Campos PH, Sanabe ME, Rodrigues JA, Duarte DA, Santos MT, Guaré RO, et al. Different bacterial models for in vitro induction of non-cavitated enamel caries-like lesions: microhardness and polarized light miscroscopy analyses. Microsc Res Tech. 2015;78(6):444-51. doi: 10.1002/jemt.22493
Steiner-Oliveira C, Maciel FA, Rodrigues LK, Napimoga MH, Pimenta LA, Höfling JF, et al. An in vitro microbial model for producing caries-like lesions on enamel. Braz J Oral Sci. 2016;6(22):1392-6. doi: 10.20396/bjos.v6i22.8642998
ten Cate JM, Duijsters PP. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res.1982;16(3):201-10. doi: 10.1159/000260599
Conrads G, de Soet JJ, Song L, Henne K, Sztajer H, Wagner-Döbler I, et al. Comparing the cariogenic species Streptococcus sobrinus and S. mutans on whole genome level. J Oral Microbiol. 2014;6:26189. doi: 10.3402/jom.v6.26189
Banas JA, Drake DR. Are the mutans streptococci still considered relevant to understanding the microbial etiology of dental caries? BMC Oral Health. 2018;18(1):129. doi: 10.1186/s12903-018-0595-2
Nascimento MM, Lemos JA, Abranches J, Gonçalves RB, Burne RA. Adaptive acid tolerance response of Streptococcus sobrinus. J Bacteriol. 2004;186(19):6383-90. doi: 10.1128/JB.186.19.6383-6390.2004
Saraithong P, Pattanaporn K, Chen Z, Khongkhunthian S, Laohapensang P, Chhun N, et al. Streptococcus mutans and Streptococcus sobrinus colonization and caries experience in 3- and 5-year-old Thai children. Clin Oral Investig. 2015;19(8):1955-64. doi: 10.1007/s00784-015-1437-0.
Simón-Soro A, Belda-Ferre P, Cabrera-Rubio R, Alcaraz LD, Mira A. A tissue-dependent hypothesis of dental caries. Caries Res. 2013;47(6):591-600. doi: 10.1159/000351663
Rupf S, Merte K, Eschrich K, Kneist S. Streptococcus sobrinus in children and its influence on caries activity. Eur Arch Paediatr Dent. 2006;7(1):17-22. doi: 10.1007/BF03320810
Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90(3):294-303. doi: 10.1177/0022034510379602
Reis AC, Bezerra DD, Hart-Chú EN, Stipp RN, Guedes SFF, Neves BG, et al. Quantification and gene expression of Lactobacillus casei group species associated with dentinal lesions in early childhood caries. Saudi Dent J. 2021;33(2):69-77. doi: 10.1016/j.sdentj.2020.01.006
Gilbert K, Joseph R, Vo A, Patel T, Chaudhry S, Nguyen U, et al. Children with severe early childhood caries: streptococci genetic strains within carious and white spot lesions. J Oral Microbiol. 2014;6. doi: 10.3402/jom.v6.25805
Caufield PW, Schön CN, Saraithong P, Li Y, Argimón S. OralLactobacilli and dental caries: a model for niche adaptation in humans. J Dent Res. 2015;94(9 Suppl):110S-8S. doi: 10.1177/0022034515576052
Eiampongpaiboon T, Chung WO, Bryers JD, Chung KH, Chan DC. Antibacterial activity of gold-titanates on Gram-positive cariogenic bacteria. Acta Biomater Odontol Scand. 2015;1(2-4):51-8. doi: 10.3109/23337931.2015.1084883
Ando M, Eckert GJ, Stookey GK, Zero DT. Effect of imaging geometry on evaluating natural white-spot lesions using quantitative light-induced fluorescence. Caries Res. 2004;38(1):39-44. doi: 10.1159/000073919
Silverstone LM. Observations on the dark zone in early enamel caries and artificial caries-like lesions. Caries Res. 1967;1(3):260-74.
Silverstone LM, Poole DF. The effect of saliva and calcifying solutions upon the histological appearance of enamel caries. Caries Res. 1968;2(1):87-93. doi: 10.1159/000259547
Sousa FB, Soares JD, Vianna SS. Natural enamel caries: a comparative histological study on biochemical volumes. Caries Res. 2013;47(3):183-92. doi: 10.1159/000345378. Erratum in: Caries Res. 2013;47(5):428.
Mohanty B, Dadlani D, Mahoney D, Mann AB. Characterizing and identifying incipient carious lesions in dental enamel using micro-Raman spectroscopy. Caries Res. 2013;47(1):27-33. doi: 10.1159/000342432
Khalid M, Bora T, Ghaithi AA, Thukral S, Dutta J. Raman Spectroscopy detects changes in bone mineral quality and collagen cross-linkage in Staphylococcus infected human bone. Sci Rep. 2018;8(1):9417. doi: 10.1038/s41598-018-27752-z
Das Gupta S, Killenberger M, Tanner T, Rieppo L, Saarakkala S, Heikkilä J, et al. Mineralization of dental tissues and caries lesions detailed with Raman microspectroscopic imaging. Analyst. 2021;146(5):1705-13. doi: 10.1039/d0an01938k
Penel G, Delfosse C, Descamps M, Leroy G. Composition of bone and apatitic biomaterials as revealed by intravital Raman microspectroscopy. Bone. 2005;36(5):893-901. doi: 10.1016/j.bone.2005.02.012
Sadyrin E, Swain M, Mitrin B, Rzhepakovsky I, Nikolaev A, Irkha V, et al. Characterization of enamel and dentine about a white spot lesion: mechanical properties, mineral density, microstructure and molecular composition. Nanomaterials (Basel). 2020;10(9):1889. doi: 10.3390/nano10091889
Shin NY, Yamazaki H, Beniash E, Yang X, Margolis SS, Pugach MK, et al. Amelogenin phosphorylation regulates tooth enamel formation by stabilizing a transient amorphous mineral precursor. J Biol Chem. 2020;295(7):1943-59. doi: 10.1074/jbc.RA119.010506
Featherstone JD, ten Cate JM, Shariati M, Arends J. Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res. 1983;17(5):385-91. doi: 10.1159/000260692
Lynch RJ, Mony U, ten Cate JM. Effect of lesion characteristics and mineralizing solution type on enamel remineralization in vitro. Caries Res. 2007;41(4):257-62. doi: 10.1159/000101914
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Journal of Applied Oral Science

This work is licensed under a Creative Commons Attribution 4.0 International License.
Todo o conteúdo do periódico, exceto onde está identificado, está licenciado sob uma Licença Creative Commons do tipo atribuição CC-BY.

