Acinetobacter baumannii as an oro-dental pathogen: a red alert!!
DOI:
https://doi.org/10.1590/1678-7757-2023-0382Keywords:
Acinetobacter baumannii, Oral health, Virulence, Biofilm, Oral cancerAbstract
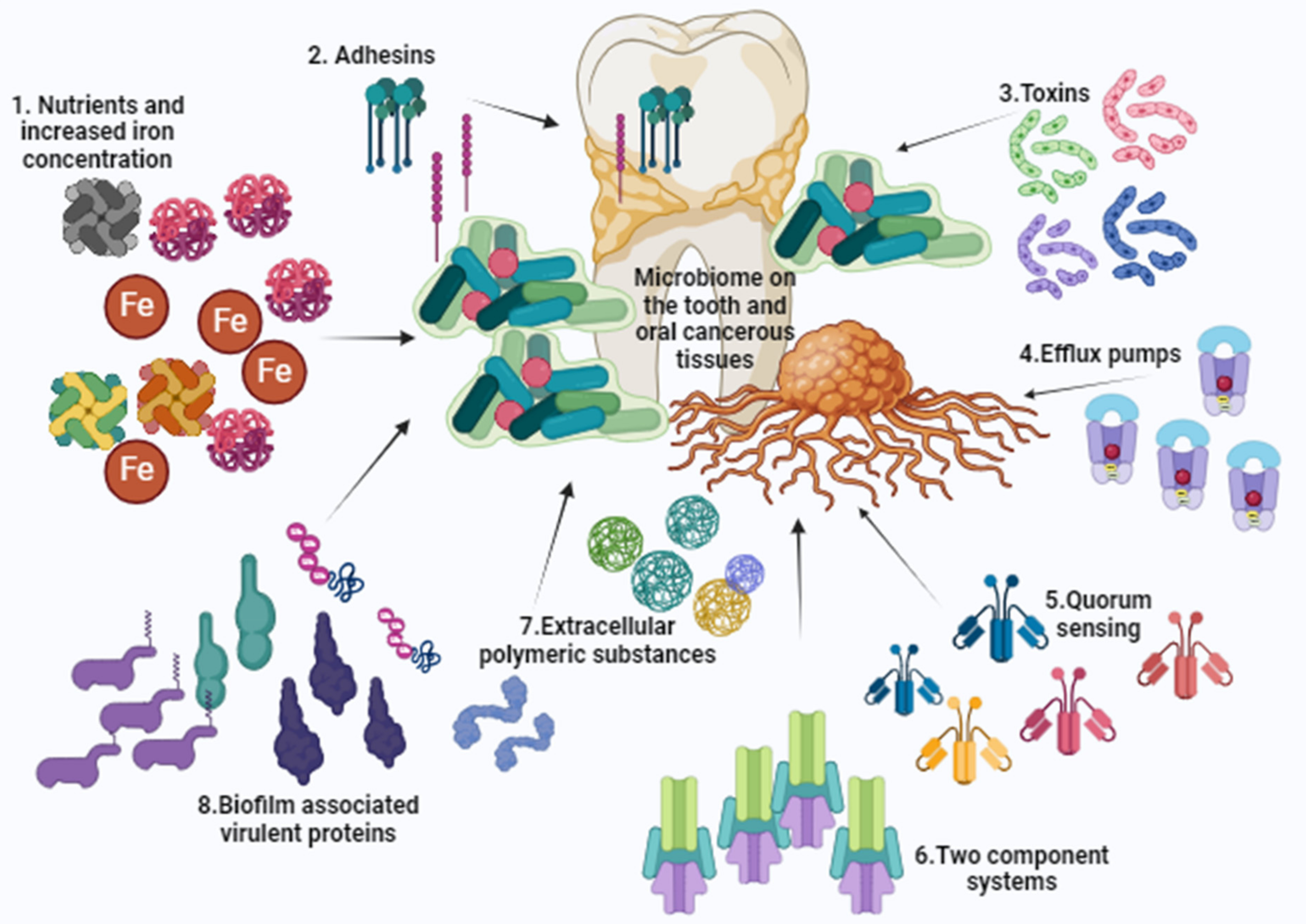
Objectives: This review highlights the existence and association of Acinetobacter baumannii with the oro-dental diseases, transforming this systemic pathogen into an oral pathogen. The review also hypothesizes possible reasons for the categorization of this pathogen as code blue due to its stealthy entry into the oral cavity. Methodology: Study data were retrieved from various search engines reporting specifically on the association of A. baumannii in dental diseases and tray set-ups. Articles were also examined regarding obtained outcomes on A. baumannii biofilm formation, iron acquisitions, magnitude of antimicrobial resistance, and its role in the oral cancers. Results: A. baumannii is associated with the oro-dental diseases and various virulence factors attribute for the establishment and progression of oro-mucosal infections. Its presence in the oral cavity is frequent in oral microbiomes, conditions of impaired host immunity, age related illnesses, and hospitalized individuals. Many sources also contribute for its prevalence in the dental health care environment and the presence of drug resistant traits is also observed. Its association with oral cancers and oral squamous cell carcinoma is also evident. Conclusions: The review calls for awareness on the emergence of A. baumannii in dental clinics and for the need for educational programs to monitor and control the sudden outbreaks of such virulent and resistant traits in the dental health care settings.
Downloads
References
Joly-Guillou ML. Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect. 2005;11(11):868-73. doi: 10.1111/j.1469-0691.2005.01227.x
Bianca Badescu, Valentina Buda, Mirabela Romanescu, Adelina Lombrea, Corina Danciu, Olivia Dalleur. Current state of knowledge regarding WHO critical priority pathogens: mechanisms of resistance and proposed solutions through candidates such as essential oils. Plants. 2022;11(14):11141789. doi: 10.3390/plants11141789
Zarrilli R, Pournaras S, Giannouli M, Tsakris A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int J Antimicrob Agents. 2013;41(1):11-9. doi: 10.1016/j.ijantimicag.2012.09.008
Brito LC, Teles FR, Teles RP, França EC, Ribeiro-Sobrinho AP, Haffajee AD, et al. Use of multiple-displacement amplification and checkerboard DNA-DNA hybridization to examine the microbiota of endodontic infections. J Clin Microbiol. 2007;45(9):3039-49. doi: 10.1128/JCM.02618-06
Silva-Boghossian CM, Souto RM, Luiz RR, Colombo AP. Association of red complex, A. actinomycetemcomitans and non-oral bacteria with periodontal diseases. Arch Oral Biol. 2011;56(9):899-906. doi: 10.1016/j.archoralbio.2011.02.009
Meinen A, Reuss A, Willrich N, Feig M, Noll I, Eckmanns T, et al. Antimicrobial resistance and the spectrum of pathogens in dental and oral-maxillofacial infections in Hospitals and Dental Practices in Germany. Front Microbiol. 2021;12:676108. doi: 10.3389/fmicb.2021.676108
Vijayashree Priyadharsini J, Smiline Girija AS, Paramasivam A. An insight into the emergence of as an oro-dental pathogen and its drug resistance gene profile - an in silico approach. Heliyon. 2018;4(12):e01051. doi: 10.1016/j.heliyon.2018.e01051
Vieira Colombo AP, Magalhães CB, Hartenbach FA, Martins do Souto R, Maciel da Silva-Boghossian C. Periodontal-disease-associated biofilm: a reservoir for pathogens of medical importance. Microb Pathog. 2016;94:27-34. doi: 10.1016/j.micpath.2015.09.009
Zaatout N. Presence of non-oral bacteria in the oral cavity. Arch Microbiol. 2021;203(6):2747-60. doi: 10.1007/s00203-021-02300-y
Gregorczyk-Maga I, Fiema M, Kania M, Kędzierska J, Jachowicz E, Romaniszyn D, et al. Cultivable oral bacteriota dysbiosis in mechanically ventilated COVID-19 patients. Front Microbiol. 2022;13:1013559. doi: 10.3389/fmicb.2022.1013559
Zawadzki PJ, Perkowski K, Starościak B, Baltaza W, Padzik M, Pionkowski K, et al. Identification of infectious microbiota from oral cavity environment of various population group patients as a preventive approach to human health risk factors. Ann Agric Environ Med. 2016;23(4):566-69. doi: 10.5604/12321966.1226847
Van Winkelhoff AJ, Rurenga P, Wekema-Mulder GJ, Singadji ZM, Rams TE. Non-oral Gram-negative facultative rods in chronic periodontitis microbiota. Microb Pathog. 2016;94:117-22. doi: 10.1016/j.micpath.2016.01.020
Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745-59. doi: 10.1038/s41579-018-0089-x
Jakovac S, Goić-Barišić I, Pirija M, Kovačić A, Hrenović J, Petrović T, et al. Molecular characterization and survival of carbapenem-resistant acinetobacter baumanniiisolated from hospitalized patients in Mostar, Bosnia and Herzegovina. Microbial Drug Resistance. 2021;27(3):383-90. doi: 10.1089/mdr.2020.0163
Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann Periodontol. 2003;8(1):54-69. doi: 10.1902/annals.2003.8.1.54.
Souto R, Silva-Boghossian CM, Colombo AP. Prevalence of Pseudomonas aeruginosa and Acinetobacter spp. in subgingival biofilm and saliva of subjects with chronic periodontal infection. Braz J Microbiol. 2014;45(2):495-501. doi: 10.1590/s1517-83822014000200017
Le MN, Kayama S, Yoshikawa M, Hara T, Kashiyama S, Hisatsune J, et al. Oral colonisation by antimicrobial-resistant Gram-negative bacteria among long-term care facility residents: prevalence, risk factors, and molecular epidemiology. Antimicrob Resist Infect Control. 2020;9(1):45. doi: 10.1186/s13756-020-0705-1
Chen P, Wu H, Yao H, Zhang J, Fan W, Chen Z, et al. Multi-omics analysis reveals the systematic relationship between oral homeostasis and chronic sleep deprivation in rats. Front Immunol. 2022;13:847132. doi: 10.3389/fimmu.2022.847132
Jain V, Baraniya D, El-Hadedy DE, Chen T, Slifker M, Alakwaa F. Integrative metatranscriptomic analysis reveals disease-specific microbiome - host interactions in oral squamous cell carcinoma. Cancer Res Commun. 2023;3(5):807-20. doi: 10.1158/2767-9764.CRC-22-0349
Sedghi L, DiMassa V, Harrington A, Lynch SV, Kapila YL. The oral microbiome: role of key organisms and complex networks in oral health and disease. Periodontol 2000. 2021;87(1):107-31. doi: 10.1111/prd.12393
Richards AM, Abu Kwaik Y, Lamont RJ. Code blue: Acinetobacter baumannii, a nosocomial pathogen with a role in the oral cavity. Mol Oral Microbiol. 2015;30(1): 2-15. doi:10.1111/omi.12072
Souto R, Silva-Boghossian CM, Colombo AP. Prevalence of Pseudomonas aeruginosa and Acinetobacter spp. in subgingival biofilm and saliva of subjects with chronic periodontal infection. Braz J Microbiol. 2014;45(2):495-501. doi: 10.1590/s1517-83822014000200017
Miller DP, Wang Q, Weinberg A, Lamont RJ. Transcriptome analysis of Porphyromonas gingivalis and Acinetobacter baumannii in polymicrobial communities. Mol Oral Microbiol. 2018;33(5):364-77. doi: 10.1111/omi.12238
Perera D, Kleinstein SE, Hanson B, Hasturk H, Eveloff R, Freire M, et al. Impaired host response and the presence of in the serum microbiome of type-II diabetic patients. iScience. 2021;24(1):101941. doi: 10.1016/j.isci.2020.101941
Anju VT, Busi S, Imchen M, Kumavath R, Mohan MS, Salim SA, et al. Polymicrobial infections and biofilms: clinical significance and eradication strategies. Antibiotics (Basel). 2022;11(12):1731. doi: 10.3390/antibiotics11121731
Miller DP, Wang Q, Weinberg A, Lamont RJ. Transcriptome analysis of Porphyromonas gingivalis and Acinetobacter baumannii in polymicrobial communities. Molecular oral microbiology. 2018;33(5):364-77. doi: 10.1111/omi.12238
Zaatout N. Presence of non-oral bacteria in the oral cavity. Arch Microbiol. 2021;203:2747-60. doi: 10.1007/s00203-021-02300-y
Silva-Boghossian CM, Souto RM, Luiz RR, Colombo AP. Association of red complex, A. actinomycetemcomitans and non-oral bacteria with periodontal diseases. Arch Oral Biol. 2011;56(9):899-906. doi: 10.1016/j.archoralbio.2011.02.009
Didilescu AC, Skaug N, Marica C, Didilescu C. Respiratory pathogens in dental plaque of hospitalized patients with chronic lung diseases. Clin Oral Investig. 2005;9(3):141-7. doi: 10.1007/s00784-005-0315-6
Heo SM, Haase EM, Lesse AJ, Gill SR, Scannapieco FA. Genetic relationships between respiratory pathogens isolated from dental plaque and bronchoalveolar lavage fluid from patients in the intensive care unit undergoing mechanical ventilation. Clin Infect Dis. 2008;47(12):1562-70. doi: 10.1086/593193
Tada A, Hanada N. Opportunistic respiratory pathogens in the oral cavity of the elderly. FEMS Immunol Med Microbiol. 2010;60(1):1-17. doi: 10.1111/j.1574-695X.2010.00709.x
Pereira TD, Travassos DV, Silva RC, Nunes LF, Santos ME, Lanza CR, et al. Acinetobacter baumannii orofacial cellulitis: report of 2 cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127(6):e118-e122. doi: 10.1016/j.oooo.2019.02.012
Duman Y, Ersoy Y, Tanriverdi ES, Otlu B, Toplu SA, Gözükara Bağ HG, et al. Oral colonization of in intensive care units: risk factors, incidence, molecular epidemiology, association with the occur of pneumonia and sepsis, and infection control measures. Iran J Basic Med Sci. 2022;25(2):239-44. doi: 10.22038/IJBMS.2022.59713.13243
Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis. 2008;8(12):751-62. doi: 10.1016/S1473-3099(08)70279-2
Raut S, Rijal KR, Khatiwada S, Karna S, Khanal R, Adhikari J, et al. Trend and characteristics of infections in patients attending Universal College of Medical Sciences, Bhairahawa, Western Nepal: a longitudinal study of 2018. Infect Drug Resist. 2020;13:1631-41. doi: 10.2147/IDR.S257851
Alp E, Coruh A, Gunay GK, Yontar Y, Doganay M. Risk factors for nosocomial infection and mortality in burn patients: 10 years of experience at a university hospital. J Burn Care Res. 2012;33(3):379-85. doi: 10.1097/BCR.0b013e318234966c
Gao L, Xu T, Huang G, Jiang S, Gu Y, Chen F. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell. 2018;9(5):488-500. doi: 10.1007/s13238-018-0548-1
Al-Khafaji SA, AL-Zreejaw SZ, Abed SM, Kadhim KH. comparison the pathogenicity, biofilm and adhesion activity of acinetobacter baumannii isolated from patients using thermoplastic retainer with other oral isolates. Int J Drug Deliv Technol. 2022;12(03):1191-95. doi: 10.25258/ijddt.12.3.45
Alagl AS, Madi M, Bedi S, Al Onaizan F, Al-Aql ZS. The effect of Er,Cr:YSGG and diode laser applications on dental implant surfaces contaminated with acinetobacter baumannii and pseudomonas aeruginosa. Materials. 2019;12(13);2073-80. doi:10.3390/ma12132073
Williams HN, Falkler WA Jr, Hasler JF. Acinetobacter contamination of laboratory dental pumice. J Dent Res. 1983;62(10):1073-75. doi: 10.1177/00220345830620101401
Umezawa K, Asai S, Ohshima T, Iwashita H, Ohashi M, Sasaki M, et al. Outbreak of drug-resistant Acinetobacter baumannii ST219 caused by oral care using tap water from contaminated hand hygiene sinks as a reservoir. Am J Infect Control. 2015;43(11):1249-51. doi: 10.1016/j.ajic.2015.06.016
Behbehani M, McDonald A, Nair SP, Green IM. Susceptibility of Pseudomonas aeruginosa and Acinetobacter baumannii biofilms grown on denture acrylic to denture-cleaning agents and sonication. bioRxiv. 2022;9. doi:10.1101/2022.09.21.508680
Sumi Y, Miura H, Sunakawa M, Michiwaki Y, Sakagami N. Colonization of denture plaque by respiratory pathogens in dependent elderly. Gerodontology. 2002;19(1):25-29. doi: 10.1111/j.1741-2358.2002.00025.x
Levy SB. Active efflux, a common mechanism for biocide and antibiotic resistance. Symp Ser Soc Appl Microbiol. 2002;(31):65S - 71S. doi.org/10.1046/j.1365-2672.92.5s1.4.x
Kim SK, Lee JH. Biofilm dispersion in Pseudomonas aeruginosa. J Microbiol. 2016;54(2):71-85. doi: 10.1007/s12275-016-5528-7
Honore PM, Djimafo P, Redant S, Attou R, Labeau S. Contamination of antimicrobial-resistant bacteria on toothbrushes used with mechanically ventilated patients: a cross sectional study. Intensive Crit Care Nurs. 2022;70:103229. doi: 10.1016/j.iccn.2022.103226
Le MN, Kayama S, Yoshikawa M, Hara T, Kashiyama S, Hisatsune J. Oral colonisation by antimicrobial-resistant Gram-negative bacteria among long-term care facility residents: prevalence, risk factors, and molecular epidemiology. Antimicrob Resist Infect Control. 2020;9(1):45. doi: 10.1186/s13756-020-0705-1
J Appl Oral Sci.2024;32:e202303829/948- Hendrickx L, Hausner M, Wuertz S. Natural genetic transformation in monoculture Acinetobacter sp. strain BD413 biofilms. Appl Environ Microbiol. 2003;69(3):1721-7. doi: 10.1128/AEM.69.3.1721-1727.2003
Anjali K, Arun AB, Bastian TS, Parthiban R, Selvamani M, Adarsh H. Oral microbial profile in oral cancer patients before and after radiation therapy in a cancer care center: a prospective study. J Oral Maxillofac Pathol. 2020;24(1):117-24. doi: 10.4103/jomfp.JOMFP_213_19
Bakhti SZ, Latifi-Navid S. Oral microbiota and Helicobacter pylori in gastric carcinogenesis: what do we know and where next? BMC Microbiol. 2021;21(1):71. doi: 10.1186/s12866-021-02130-4
Yang J, He P, Zhou M, Li S, Zhang J, Tao X, et al. Variations in oral microbiome and its predictive functions between tumorous and healthy individuals. J Med Microbiol. 2022;71(8). doi:10.1099/jmm.0.001568
Sidi Omar SF, Ngui R, Ab Rahman SZ, Foo JC, Wang QY, Hassan NA, et al. Oral bacteria detection among children with cancer in a tertiary teaching hospital in Kuala Lumpur, Malaysia. Trop Biomed. 2021;38(3):276-82. doi: 10.47665/tb.38.3.068
Bartochowska A, Tomczak H, Wierzbicka M. Acinetobacter: an enemy after head and neck cancer operations with microvascular free flap reconstruction? Surg Infect . 2021;22(4):442-6. doi: 10.1089/sur.2020.214
Tuominen H, Rautava J. Oral microbiota and cancer development. Pathobiology. 2021;88(2):116-26. doi: 10.1159/000510979
Gentile V, Frangipani E, Bonchi C, Minandri F, Runci F, Visca P. Iron and Acinetobacter baumannii Biofilm Formation. Pathogens. 2014;3(3):704-19. doi: 10.3390/pathogens3030704
Arthur RA, Santos Bezerra R, Ximenez JP, Ximenez JP, Merlin BL, Andrade Morraye R, et al. Microbiome and oral squamous cell carcinoma: a possible interplay on iron metabolism and its impact on tumor microenvironment. Braz J Microbiol. 2021;52(3):1287-902. doi: 10.1007/s42770-021-00491-6
Newman T, Krishnan LP, Lee J, Adami GR. Microbiomic differences at cancer-prone oral mucosa sites with marijuana usage. Sci Rep. 2019;9(1):12697. doi: 10.1038/s41598-019-48768-z
Hooper SJ, Crean SJ, Lewis MA, Spratt DA, Wade WG, Wilson MJ. Viable bacteria present within oral squamous cell carcinoma tissue. J Clin Microbiol. 2006;44(5):1719-25. doi: 10.1128/JCM.44.5.1719-1725.2006
He X, Lu F, Yuan F, Jiang D, Zhao P, Zhu J,et al. Biofilm formation caused by clinical Acinetobacter baumannii isolates is associated with overexpression of the AdeFGH Efflux Pump. Antimicrob Agents Chemother. 2015;59(8):4817-25. doi: 10.1128/AAC.00877-15
Lin L, Tan B, Pantapalangkoor P, Ho T, Baquir B, Tomaras A, et al. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. MBio. 2012;3(5):e00312-12. doi:10.1128/mBio.00312-12
Luke NR, Sauberan SL, Russo TA, Beanan JM, Olson R, Loehfelm TW, et al. Identification and characterization of a glycosyltransferase involved in Acinetobacter baumannii lipopolysaccharide core biosynthesis. Infect Immun. 2010;78(5):2017-23. doi: 10.1128/IAI.00016-10
Islam MM, Kim K, Lee JC, Shin M. LeuO, a LysR-type transcriptional regulator, is involved in biofilm formation and virulence of Acinetobacter baumannii. Front Cell Infect Microbiol. 2021;11(11):738706.
Zhang Z, Yang J, Feng Q, Chen B, Li M, Liang C, et al. Compositional and functional analysis of the microbiome in tissue and saliva of oral squamous cell carcinoma. Front Microbiol. 2019;10:1439. doi: 10.3389/fmicb.2019.01439
Tomaras AP, Dorsey CW, Edelmann R, Actis LA. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology.2003;149(12):3473-84. doi: 10.1099/mic.0.26541-0
Nie D, Hu Y, Chen Z, Li M, Hou Z, Luo X, et al. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J Biomed Sci.2020;27(1):26. doi: 10.1186/s12929-020-0617-7
Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295(5559):1487. doi: 10.1126/science.295.5559.1487
Li YH, Tian X. Quorum sensing and bacterial social interactions in biofilms. Sensors. 2012;12(3):2519-38. doi: 10.3390/s120302519
Zhong S, He S. Quorum sensing inhibition or quenching in Acinetobacter baumannii: the novel therapeutic strategies for new drug development. Front. Microbiol.2021;12:558003. doi: 10.3389/fmicb.2021.558003
Choi AH, Slamti L, Avci FY, Pier GB, Maira-Litrán T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-β-1-6-N-acetylglucosamine, which is critical for biofilm formation. J Bacteriol. 2009;191(19):5953-63. doi: 10.1128/JB.00647-09
Loehfelm TW, Luke NR, Campagnari AA. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J Bacteriol. 2008;190(3):1036-44. doi: 10.1128/JB.01416-07
Abdi SN, Ghotaslou R, Asgharzadeh M, Mehramouz B, Hasani A, Baghi HB, et al. AdeB efflux pump gene knockdown by mRNA mediated peptide nucleic acid in multidrug resistance Acinetobacter baumannii. Microb Pathog. 2020;139:103825. doi: 10.1016/j.micpath.2019.103825
Sahu PK, Iyer PS, Barage SH, Sonawane KD, Chopade BA. Characterization of the algC gene expression pattern in the multidrug resistant Acinetobacter baumannii AIIMS 7 and correlation with biofilm development on abiotic surface. Sci World J. 2014;2014:593546. doi: 10.1155/2014/593546
Jaisankar AI, Girija AS, Gunasekaran S, Priyadharsini JV. Molecular characterization of csgA gene among ESBL strains of A. baumannii and targeting with essential oil compounds from Azadirachta indica. J King Saud Univ-Sci. 2020;32(8):3380-7. doi: 10.1016/j.jksus.2020.09.025
Draughn GL, Milton ME, Feldmann EA, Bobay BG, Roth BM, Olson AL. The structure of the biofilm-controlling response regulator BfmR from Acinetobacter baumannii reveals details of its DNA-binding mechanism. J Mol Biol. 2018;430(6):806-21. doi: 10.1016/j.jmb.2018.02.002
Richmond GE, Evans LP, Anderson MJ, Wand ME, Bonney LC, Ivens A, et al. The Acinetobacter baumannii two-component system AdeRS regulates genes required for multidrug efflux, biofilm formation, and virulence in a strain-specific manner. mBio.2016;7(2):e00430-16. doi: 10.1128/mBio.00430-16
Abdullah RM, Ahmed RZ. Genotype detection of fimH gene of Acinetobacter baumannii isolated from different clinical cases. J Biotech Res Center.2019;13(1):23-8.
Zeighami H, Valadkhani F, Shapouri R, Samadi E, Haghi F. Virulence characteristics of multidrug resistant biofilm forming Acinetobacter baumannii isolated from intensive care unit patients. BMC Infect. Dis. 2019;19(1):1-9. doi: 10.1186/s12879-019-4272-0
Kanaan MH, Khashan HT. Molecular typing, virulence traits and risk factors of pandrug-resistant Acinetobacter baumannii spread in intensive care unit centers of Baghdad city. Iraq. Rev Med Microbiol. 2021;33(1):51-5. doi: 10.1097/MRM.0000000000000282
Downloads
Published
Versions
- 2024-05-13 (2)
- 2024-05-13 (1)
Issue
Section
License
Copyright (c) 2024 Journal of Applied Oral Science

This work is licensed under a Creative Commons Attribution 4.0 International License.
Todo o conteúdo do periódico, exceto onde está identificado, está licenciado sob uma Licença Creative Commons do tipo atribuição CC-BY.

